Serving patients
and demonstrating
leadership
by providing customers
with engaging service
and product excellence

Dedicated to providing
advanced tissue-repair products
for improving patient outcomes worldwide

Derived from naturally occurring
extracellular matrices
such as porcine small intestinal submucosa (SIS)

SIS supports site-specific tissue remodeling.
Restoring tissue. Improving life.
SIS supports site-specific tissue remodeling.




Restoring tissue. Improving life.
Developing advanced biomaterials
Technologies



From Head & Neck to Extremities
Products
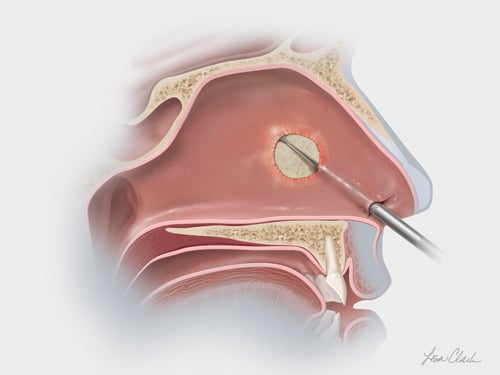
Head & Neck
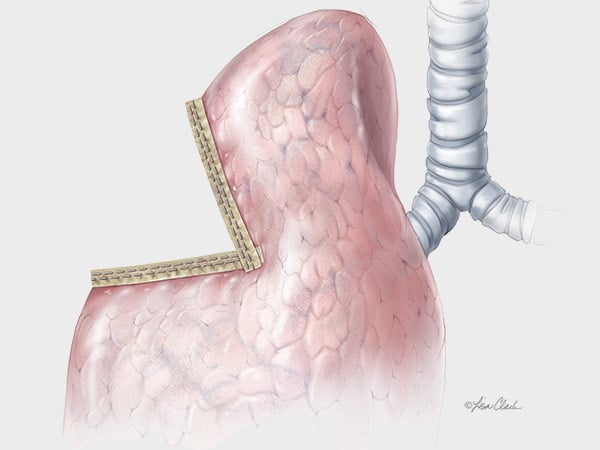
Chest & Abdomen
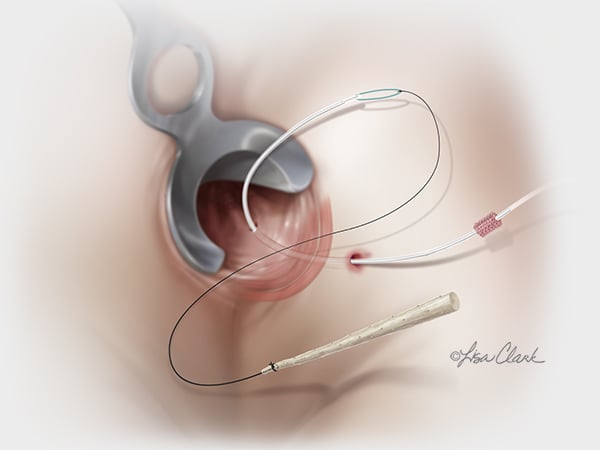
Pelvis
Extremities
Cook Biotech Inc.
About
Cook Biotech Inc. was established in 1995 to develop medical solutions based on the regenerative properties of extracellular matrix materials discovered at Purdue University.
More than 25 years later, our proprietary technologies and manufacturing processes have produced more than 6 million advanced tissue-repair products for global distribution.
We actively seek and welcome partnerships with researchers, distributors, start-up medical companies, and established medical companies to further our mission of serving patients.
We are proud to be part of Cook Group Inc., a global family of socially responsible companies.